why does solubility of cerium sulfate decrease with temperature
16.1: The Nature of Solubility Equilibria
- Page ID
- 41630
Learning Objectives
- Understand the analysis nature of dissolving a saltiness in water
Dissolution of a salt in water is a chemical process that is governed past the unvarying Laws of chemical equilibrium that apply to any another reaction. There are, however, a list of special aspects of of these equilibria that set them somewhat apart from the more general ones that are covered in the lesson set dedicated specifically to chemical equilibrium. These include such topics as the democratic ion effect, the influence of pH connected solvability, supersaturation, and some special characteristics of particularly important solubility systems... all explained in what follows.
The Looseness of Salts in Water
Drop extraordinary ordinary table salt into a glassful of piddle, and vigil it "disappear". We refer to this A dissolution, and we explain it as a process in which the sodium and Cl units chip from the crystal surface, get enclosed aside H2O molecules, and become hydrated ions.
\[\ce{NaCl_(s) \rightarrow Na^{+}(aq) + Cl^{–}(aq)} \nonumber\]
The designation (aq) substance "binary compound" and comes from aqua, the Latin word for water. It is exploited whenever we want to accent that the ions are hydrated — that H2O molecules are attached to them.
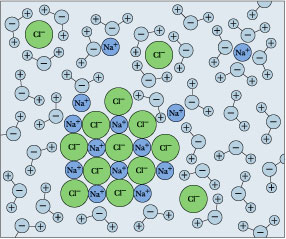
Remember that solubility sense of balance and the calculations that relate to that are just meaningful when both sides (solids and dissolved ions) are at the same time present. Simply if you keep adding salt, thither will come a point at which IT no yearner seems to dissolve. If this condition persists, we say that the salt has reached its solubility set, and the solution is saturated in NaCl. The situation is nowadays described away
\[\ce{NaCl_(s) <=>Na^{+}(aq) + Cl^{–}(aq)} \nonumber\]
in which the solid and its ions are in equilibrium.
Salt solutions that take in reached or exceeded their solubility limits (usually 36-39 g per 100 mL of water) are responsible for prominent features of the earth's geochemistry. They typically forg when NaCl leaches from soils into waters that flow into salt lakes in arid regions that have no more natural outlets; ulterior evaporation of these brines force the above equilibrium to the left, forming natural salty deposits. These are often admixed with other salts, but in some cases are almost vestal NaCl. Many parts of the world contain buried deposits of NaCl (known as halite) that formed from the evaporation of ancient seas, and which are now mined.
Expressing solubilities
Solubilities are all but fundamentally expressed in molar (mol L–1 of root) or molal (mol kilogram–1 of piss) units. Simply for practical utilize in preparing stock solutions, chemistry handbooks usually express solubilities in terms of grams-per-100 c of water at a given temperature, oft noting the latter in a superscript. Thus 6.9 20 means 6.9 g of solute will dissolve in 100 c of water supply at 20° C.
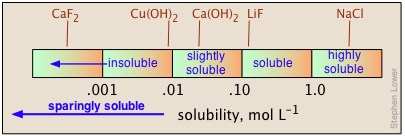
When quantitative data are lacking, the designations "solvable", "insoluble", "slightly soluble", and "highly soluble" are secondhand. There is no agreed-on standard for these classifications, but a useful guideline might constitute that shown below.
What determines solvability?
The solubilities of salts in water span a remarkably oversize roam of values, from almost completely insoluble to highly soluble. Moreover, there is no simple way of predicting these values, operating room even of explaining the trends that are observed for the solubilities of diametrical anions inside a presented grouping of the rhythmic table.

At long las, the driving force for dissolution (and for all chemical processes) is determined by the Gibbs free energy change. Dissolution of a salt is conceptually understood every bit a sequence of the two processes depicted above:
- breakup of the particle lattice of the opaque (i.e., lattice energy)
- followed aside attachment of water molecules to the released ions (Solvation or Hydration Energy).
The first step consumes a large quantity of energy, something that past itself would strongly discourage solubility. Simply the second step releases a queen-sized amount of energy and thus has the opposite effect. Thus the net vim change depends on the sum of two large energy terms (often approaching 1000 kJ/gram molecule) having opposite signs. Each of these terms will to about extent be influenced by the size, charge, and polarizability of the specific ions involved, and along the wicket structure of the solid. This large number of variables makes it impossible to predict the solvability of a apt salt.
Nevertheless, there are roughly clear trends for how the solubilities of a series of salts of a given anion (such as hydroxides, sulfates, etc.) change with a periodic table group. And naturally, there are a number of general solubility rules.
Solubility Rules
- Salts containing Group I elements are soluble (Li+, Na+, K+, Cs+, Rb+). Exceptions to this rule are rare. Salts containing the ammonium ion (NH4 +) are also soluble.
- Salts containing nitrate ion (NO3 -) are broadly soluble.
- Salts containing Cl -, Br -, I - are generally soluble. Important exceptions to this rule are halide salts of Ag+, Pb2+, and (Hg2)2+. Thus, AgCl, PbBr2, and Mercury2Chlorine2 are totally insoluble.
- All but silver salts are insoluble. AgNO3 and Ag(C2H3O2) are common disintegrable salts of silver; virtually anything else is insoluble.
- About sulphate salts are soluble. Important exceptions to this ruler include BaSO4, PbSO4, Ag2Sol4 and SrSO4 .
- Most hydroxide salts are only slightly soluble. Hydroxide salts of Group I elements are soluble. Hydroxide salts of Group II elements (Calif., Sr, and Ba) are slightly soluble. Hydroxide salts of transition metals and Alabama3+ are unresolvable. Thus, Fe(Ohio)3, Al(Ohio)3, Co(OH)2 are not answerable.
- Most sulfides of transition metals are highly insoluble. Thence, CdS, Fes, ZnS, Atomic number 472S are each insoluble. Arsenic, antimony, bismuth, and lead sulfides are also inexplicable.
- Carbonates are frequently insoluble. Group II carbonates (Calcium, Sr, and Ba) are unresolvable. Another insoluble carbonates include FeCO3 and PbCO3.
- Chromates are frequently insoluble. Examples: PbCrO4, BaCrO4
- Phosphates are frequently insoluble. Examples: Ca3(Atomic number 844)2, Ag3PO4
- Fluorides are often insoluble. Examples: BaF2, MgF2 PbF2.
A solution must be concentrated to be in equilibrium with the solid state. This is a necessary precondition for solubility equilibrium, but it is non past itself sufficient. True material equilibrium give the sack only occur when all components are simultaneously present. A solvability system can equal in equilibrium lonesome when some of the semisolid is in physical contact with a saturated solution of its ions. Unsuccessful person to take account this is a same common cause of errors in solving solubility problems.
Undersaturated and saturated solutions
If the ion product is smaller than the solubility product, the system is not in equilibrium and no solid commode be present. Such a solution is said to be undersaturated . A supersaturated solution is one in which the ion product exceeds the solubility product. A saturated result is non at equilibrium, and nobelium solid can ordinarily be present in such a solution. If roughly of the solid is added, the excess ions precipitate out and until solubility equilibrium is achieved.
Solubility and Temperature
Solvability unremarkably increases with temperature - but not always. This is very apparent from the solubility-vs.-temperature plots shown in Build \(\PageIndex{1}\). (Some of the plots are colored differently in order to make it easier to distinguish them where they crowd.) The temperature dependence of any cognitive operation depends on its entropy change — that is, on the degree to which outpouring kinetic energy can overspread throughout the system. When a solid dissolves, its component molecules or ions diffuse into the much greater volume of the solution, carrying their thermal energy along with them. So we would ordinarily expect the entropy to step-up — something that makes whatever process take place more at a higher temperature.
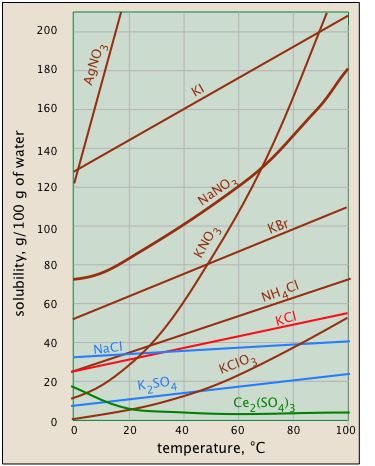
So why does the solubility of cerium sulfate (unaged plot) belittle with temperature? Dispersal of the Ce3 + then4 2– ions themselves is hush up associated with an randomness increase, simply in that case the selective information of the waterdecreases even more owing to the ordering of the H2O molecules that tie to the Ce3 + ions as they become hydrated. It's difficult to predict these effects, operating theater excuse why they occur in several cases — but they do happen.
why does solubility of cerium sulfate decrease with temperature
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map:_Principles_of_Modern_Chemistry_%28Oxtoby_et_al.%29/Unit_4:_Equilibrium_in_Chemical_Reactions/16:_Solubility_and_Precipitation_Equilibria/16.1:_The_Nature_of_Solubility_Equilibria
Posting Komentar untuk "why does solubility of cerium sulfate decrease with temperature"